When I was young, many years ago, we would dress up as cowboys and Indians, soldiers and cartoon characters. We would go around to the neighbors we knew and they would tell us how cute we were and give us a simple bit of candy or gum. It seemed so innocent at the time. Now the houses are decorated as graveyards and ghosts and vampires roam the streets. We have violent movies and vampires made to look like everyday people. The more horrible the act, the more we want to see it on screen. The more devious and grotesque the better.
Halloween is not just an innocent bit of fun any longer. It is an opportunity for the enemy to get a hook into our lives and make us think it is OK to dwell on the occult. The more we sink into his darkness, the easier it is to think of it as normal and acceptable and the harder it is to see the need for the light of God in our lives. Ephesians 5:11 says "Have nothing to do with the fruitless deeds of darkness, but rather expose them. (NIV)" We need to keep our eyes on the light of His truth and not on the darkness of deception and evil.
2nd Corinthians 4:6 says "For God, who said, 'Let light shine out of darkness,' made His light shine in our hearts to give us the light of the knowledge of the glory of God in the face of Christ. (NIV)" Isn't it interesting that we cannot say Merry Christmas or Happy Easter because we might offend someone but it is OK to say Happy Halloween because no one should be offended? Bring His light into your life today and let it shine brightly to expose the darkness of reveling in the spirit of perdition that is all around us on this day. It may seem like harmless fun, but underneath it all lays the potential destruction of our spirit. Do not let it capture your soul.
Deuteronomy 29:29 - The secret things belong to the LORD our God, but the things revealed belong to us and to our children forever, that we may follow all the words of this law. (NIV)
Monday, October 31, 2011
Wednesday, October 26, 2011
Glucose - Ah, Sugar Sugar!
I love a good meal. Cooking is one of my favorite pastimes. I especially like to make cookies and cakes. I prepare a mean cheesecake! It is always a holiday favorite. But man does not live by cheesecake alone. Our cells primarily use sugar, or more specifically glucose, as food. We take in proteins, fats and carbohydrates as food or calorie sources and our body uses them as building blocks or as a means to supply or store glucose.
Glucose is a simple sugar with a chemical formula of C6H12O6. The name "glucose" comes from the Greek word glukus, meaning "sweet" and the suffix "-ose" denoting a sugar. It exists in a number of isomers but the one we utilize is D-glucose or dextrose. You might remember this as the main building block of cellulose from the Cotton-Picking Carbohydrate blog. Our bodies can not metabolize cellulose to produce glucose as ruminants (animals that chew the cud) can due to their multiple stomachs. We get our glucose from other sugars, most notably sucrose or table sugar, or from more complex carbohydrates such as starches.
In our bodies glucose is metabolized by cells either through aerobic or anaerobic respiration. Aerobic (with oxygen) is the most utilized and yields the most energy as Adenosine triphosphate or ATP. Anaerobic is only used when insufficient oxygen is available to support aerobic respiration. The complete cellular respiration reaction includes glycolysis, pyruvate decarboxylation and the Krebs Cycle. Ultimately, glucose is metabolized to just water and carbon dioxide.

Glucose metabolism and various forms of glucose during the process
Now just because glucose is our primary energy source, it is not a license to eat another donut. Our body is using glucose constantly, more or less at any moment depending on our metabolic rate. Thus when we are asleep, our metabolic rate and our use of glucose is down. When we are running a marathon, it goes up considerably. It would be impossible to supply a steady stream of glucose to our cells under all situations by just eating sugar. Our bodies need to store and release the glucose in just the right amounts and at just the right time. Thus some of the calories we eat are stored as glycogen, a protein with many branched chains of glucose molecules attached, waiting to be used as glucose. Glycogen is synthesized in the liver and muscle tissue as a storage depot for quick release glucose.
Our bodies also store fat as adipose tissue to be used as a slow release energy source. Fat in the form of triglycerides, an ester derived from glycerol and three fatty acids, can be used to generate glucose. Fat also is metabolized by the Krebs Cycle to generate ATP without glucose.
In order for our bodies to use glucose, it must get into the cell. This is the job of insulin, a protein hormone created in the pancreas and released as needed to maintain proper blood sugar concentration. Our cells can not absorb the glucose from the blood unless insulin is present. When a cell has insulin attached to its surface, the cell activates other receptors designed to absorb glucose from the bloodstream into the inside of the cell.
As you can see our bodies would not be able to go without glucose. It is the starting point for energy creation for almost all of our bodies functions. Without it, life would not be so "sweet!"
Psalm 119:103 - "How sweet are your words to my taste, sweeter than honey to my mouth! (NIV)"
Glucose is a simple sugar with a chemical formula of C6H12O6. The name "glucose" comes from the Greek word glukus, meaning "sweet" and the suffix "-ose" denoting a sugar. It exists in a number of isomers but the one we utilize is D-glucose or dextrose. You might remember this as the main building block of cellulose from the Cotton-Picking Carbohydrate blog. Our bodies can not metabolize cellulose to produce glucose as ruminants (animals that chew the cud) can due to their multiple stomachs. We get our glucose from other sugars, most notably sucrose or table sugar, or from more complex carbohydrates such as starches.
In our bodies glucose is metabolized by cells either through aerobic or anaerobic respiration. Aerobic (with oxygen) is the most utilized and yields the most energy as Adenosine triphosphate or ATP. Anaerobic is only used when insufficient oxygen is available to support aerobic respiration. The complete cellular respiration reaction includes glycolysis, pyruvate decarboxylation and the Krebs Cycle. Ultimately, glucose is metabolized to just water and carbon dioxide.
Glucose metabolism and various forms of glucose during the process
- Glucose-containing compounds are digested and taken up in the intestines, including starch, glycogen, di- and mono-saccharides.
- Glucose is stored mainly in the liver and muscles as glycogen.
- Glucose is distributed and utilized in tissues as free glucose.
Now just because glucose is our primary energy source, it is not a license to eat another donut. Our body is using glucose constantly, more or less at any moment depending on our metabolic rate. Thus when we are asleep, our metabolic rate and our use of glucose is down. When we are running a marathon, it goes up considerably. It would be impossible to supply a steady stream of glucose to our cells under all situations by just eating sugar. Our bodies need to store and release the glucose in just the right amounts and at just the right time. Thus some of the calories we eat are stored as glycogen, a protein with many branched chains of glucose molecules attached, waiting to be used as glucose. Glycogen is synthesized in the liver and muscle tissue as a storage depot for quick release glucose.
Our bodies also store fat as adipose tissue to be used as a slow release energy source. Fat in the form of triglycerides, an ester derived from glycerol and three fatty acids, can be used to generate glucose. Fat also is metabolized by the Krebs Cycle to generate ATP without glucose.
In order for our bodies to use glucose, it must get into the cell. This is the job of insulin, a protein hormone created in the pancreas and released as needed to maintain proper blood sugar concentration. Our cells can not absorb the glucose from the blood unless insulin is present. When a cell has insulin attached to its surface, the cell activates other receptors designed to absorb glucose from the bloodstream into the inside of the cell.
As you can see our bodies would not be able to go without glucose. It is the starting point for energy creation for almost all of our bodies functions. Without it, life would not be so "sweet!"
Psalm 119:103 - "How sweet are your words to my taste, sweeter than honey to my mouth! (NIV)"
Saturday, October 22, 2011
Quick Facts about the Period III Elements
There are 92 naturally occurring elements in the Periodic Table. I started this blog in June going through those elements and provided a small sampling of interesting facts about them. I also recently reviewed the first two Periods, elements Hydrogen to Neon. I thought I would hit a few more elements, those in Period III, here quickly.
Sodium - Room temperature sodium metal is soft enough that you can cut it with a butter knife.
Magnesium - A thin film of magnesium fluoride (MgF2) is applied to optical lenses to help reduce glare and reflections.

Aluminum - Rubies and Sapphires are gemstones of Aluminum Oxide (Al2O3). The different colors are caused by trace amounts of other elements such as iron, titanium, or chromium.
Natural Ruby crystals - GNUF
Silicon - To make wafers for computer chips, silicon is chemically processed until it is 99.9999% pure.
Phosphorus - As phosphate (-PO4), phosphorus is a component of DNA, RNA, and ATP (a primary biological energy source), and also the phospholipids that form cell membranes.
Sulfur - When combined with water, sulfur dioxide (SO2 - generated by burning sulfur) forms sulfurous acid (H2SO3), a weak acid that is a major component of acid rain.
Chlorine - Chlorine was used as a chemical weapon in World War I, first in 1915 by the German army and then by the Western Allies. It was not very effective because chlorine has a strong smell and is water soluble; soldiers could protect themselves from the worst of its effects by breathing through damp cloths.
Argon - Argon is used in SCUBA diving to inflate a drysuit for diving in cold water. Argon provides better insulation (1.5 times) from the cold than plain air.
Once again each successive element is different from the ones before, both in form and function. From so many small parts has the universe been made.
Hebrews 11:3 - "By faith we understand that the universe was formed at God's command, so that what is seen was not made out of what was visible. (NIV)"
Sodium - Room temperature sodium metal is soft enough that you can cut it with a butter knife.
Magnesium - A thin film of magnesium fluoride (MgF2) is applied to optical lenses to help reduce glare and reflections.
Aluminum - Rubies and Sapphires are gemstones of Aluminum Oxide (Al2O3). The different colors are caused by trace amounts of other elements such as iron, titanium, or chromium.
Natural Ruby crystals - GNUF
Silicon - To make wafers for computer chips, silicon is chemically processed until it is 99.9999% pure.
Phosphorus - As phosphate (-PO4), phosphorus is a component of DNA, RNA, and ATP (a primary biological energy source), and also the phospholipids that form cell membranes.
Sulfur - When combined with water, sulfur dioxide (SO2 - generated by burning sulfur) forms sulfurous acid (H2SO3), a weak acid that is a major component of acid rain.
Chlorine - Chlorine was used as a chemical weapon in World War I, first in 1915 by the German army and then by the Western Allies. It was not very effective because chlorine has a strong smell and is water soluble; soldiers could protect themselves from the worst of its effects by breathing through damp cloths.
Argon - Argon is used in SCUBA diving to inflate a drysuit for diving in cold water. Argon provides better insulation (1.5 times) from the cold than plain air.
Once again each successive element is different from the ones before, both in form and function. From so many small parts has the universe been made.
Hebrews 11:3 - "By faith we understand that the universe was formed at God's command, so that what is seen was not made out of what was visible. (NIV)"
Thursday, October 20, 2011
Cellulose - That Cotton-Picking Carbohydrate!
When my kids were young we went to carnivals and fairs and would often get that sticky pink web of sugar called cotton candy. It consisted of air that dissolved on your tongue with the sweet taste of spun excitement. Well it got its name because the soft fluffy appearance of the sugary stuff looks very much like an open cotton boll, the seed capsule of the cotton plant. Cotton and cotton candy have more in common than just their good looks, however. Cotton is primarily cellulose, a carbohydrate made up of thousands of glucose molecules and cotton candy is made from sucrose, a disaccharide made from glucose and fructose. 
Cotton is the soft, fluffy fiber found inside that cottonseed capsule. The cotton plant is native to tropical and subtropical regions of the world, including the Americas, Africa, and India. Cotton has been spun, woven, and dyed since prehistoric times. It clothed the people of ancient India, Egypt, and China. Cotton was domesticated over 7000 years ago but was not widely used until the invention of the Cotton Gin, a device for removing the seeds from the fibers, in 1793 by Eli Whitney. The cottonseed, which remains after the cotton is ginned, is used to produce cottonseed oil that can be consumed like any other vegetable oil. Cotton fiber is now most often spun into yarn or thread and used to make a soft, breathable cloth. It is the most widely used natural fiber in clothing today.
There are two species of cotton that account for 98% of all cotton production. They are Upland cotton (90%), native to North and Central America, and Egyptian or Pima Cotton (8%), native to South America. The remaining cotton comes from Africa and the Middle East. Although the name Egyptian cotton is sometimes associated with a higher quality product, most products bearing that name are not made with the finest cottons from Egypt. The five leading exporters of cotton are the United States, India, Brazil, Australia, and Uzbekistan. The largest importers are Korea, Taiwan, Russia, Hong Kong and Japan.
 Cotton is 91% cellulose (plus 8% water), compared to 40-50% for wood. Cellulose is a polysaccharide consisting of linked glucose units with the formula (C6H10O5)n. Cellulose from wood has typical chain lengths between 300 and 1700 glucose units; cotton and other plant fibers have chain lengths ranging from 800 to 10,000 units. Cellulose is the principal structural component of the cell wall of green plants with 33% of all plant material being made of cellulose. It is the most common organic compound on Earth.
Cotton is 91% cellulose (plus 8% water), compared to 40-50% for wood. Cellulose is a polysaccharide consisting of linked glucose units with the formula (C6H10O5)n. Cellulose from wood has typical chain lengths between 300 and 1700 glucose units; cotton and other plant fibers have chain lengths ranging from 800 to 10,000 units. Cellulose is the principal structural component of the cell wall of green plants with 33% of all plant material being made of cellulose. It is the most common organic compound on Earth.
By the mid-1800s, cotton had become the chief plantation crop in the southern United States. Cultivating and harvesting cotton was the leading occupation of slaves. Cotton remained key even after the end of the Civil War as cotton plantations required vast labor forces to hand-pick cotton. It was not until the 1950s that reliable harvesting machinery was introduced into the South.
In North America, the most economically destructive pest affecting cotton is the boll weevil. The cotton industry relies heavily on chemicals, such as herbicides, fertilizers and insecticides. Genetically modified cotton, produced by Monsanto, was developed to reduce the heavy reliance on pesticides and to resist Roundup®, used to control weeds, also sold by Monsanto. Monsanto thus controls the cotton market by selling the seeds and the chemicals used to improve the crop yields.
A very small number of farmers are now moving to organic production, and organic cotton products are available for purchase in limited numbers. Organic cotton is considered cotton from plants that are not genetically modified and are grown without the use of any synthetic fertilizers or pesticides.
Cotton has a number of "competitors" that have been developed over the last hundred years. The first was rayon, developed in France in the 1890s. Rayon is derived from natural cellulose, but requires extensive processing. A succession of synthetic fibers were then introduced by the chemical industry. Acetate fiber was developed in 1924. DuPont introduced nylon in 1936, followed by acrylic in 1944. It was not until the introduction of polyester in the early 1950s, however, that cotton came under threat of market share. While many fabrics are still made completely of cotton, some materials blend cotton with these other fibers to produce a wide variety of fabric choices. Check them out at your favorite department store soon but be sure to wait for the next sale!
"Besides, who would patch old clothing with new cloth? For the new patch would shrink and rip away from the old cloth, leaving an even bigger tear than before. Mark 2:21 (NLT)"
Cotton is the soft, fluffy fiber found inside that cottonseed capsule. The cotton plant is native to tropical and subtropical regions of the world, including the Americas, Africa, and India. Cotton has been spun, woven, and dyed since prehistoric times. It clothed the people of ancient India, Egypt, and China. Cotton was domesticated over 7000 years ago but was not widely used until the invention of the Cotton Gin, a device for removing the seeds from the fibers, in 1793 by Eli Whitney. The cottonseed, which remains after the cotton is ginned, is used to produce cottonseed oil that can be consumed like any other vegetable oil. Cotton fiber is now most often spun into yarn or thread and used to make a soft, breathable cloth. It is the most widely used natural fiber in clothing today.
There are two species of cotton that account for 98% of all cotton production. They are Upland cotton (90%), native to North and Central America, and Egyptian or Pima Cotton (8%), native to South America. The remaining cotton comes from Africa and the Middle East. Although the name Egyptian cotton is sometimes associated with a higher quality product, most products bearing that name are not made with the finest cottons from Egypt. The five leading exporters of cotton are the United States, India, Brazil, Australia, and Uzbekistan. The largest importers are Korea, Taiwan, Russia, Hong Kong and Japan.
By the mid-1800s, cotton had become the chief plantation crop in the southern United States. Cultivating and harvesting cotton was the leading occupation of slaves. Cotton remained key even after the end of the Civil War as cotton plantations required vast labor forces to hand-pick cotton. It was not until the 1950s that reliable harvesting machinery was introduced into the South.
In North America, the most economically destructive pest affecting cotton is the boll weevil. The cotton industry relies heavily on chemicals, such as herbicides, fertilizers and insecticides. Genetically modified cotton, produced by Monsanto, was developed to reduce the heavy reliance on pesticides and to resist Roundup®, used to control weeds, also sold by Monsanto. Monsanto thus controls the cotton market by selling the seeds and the chemicals used to improve the crop yields.
A very small number of farmers are now moving to organic production, and organic cotton products are available for purchase in limited numbers. Organic cotton is considered cotton from plants that are not genetically modified and are grown without the use of any synthetic fertilizers or pesticides.
Cotton has a number of "competitors" that have been developed over the last hundred years. The first was rayon, developed in France in the 1890s. Rayon is derived from natural cellulose, but requires extensive processing. A succession of synthetic fibers were then introduced by the chemical industry. Acetate fiber was developed in 1924. DuPont introduced nylon in 1936, followed by acrylic in 1944. It was not until the introduction of polyester in the early 1950s, however, that cotton came under threat of market share. While many fabrics are still made completely of cotton, some materials blend cotton with these other fibers to produce a wide variety of fabric choices. Check them out at your favorite department store soon but be sure to wait for the next sale!
"Besides, who would patch old clothing with new cloth? For the new patch would shrink and rip away from the old cloth, leaving an even bigger tear than before. Mark 2:21 (NLT)"
Sunday, October 16, 2011
Wine - More Than Just Two Buck Chuck
A good meal, coupled with a great wine, is one of life's simple pleasures. We have been enjoying wine for thousands of years but the sciences of Vinification and Oenology have blossomed only in the last several hundred years or so.
 Although it can be simply enjoyed, the process of making wine from grapes is a demanding process. It is a delicate dance of storage, temperature, yeast and oxygen (or lack thereof) with time. The flavor and the alcoholic strength depends greatly on the control the winemaker exercises over these variables. The primary reaction is the conversion of the natural sugars in the grapes by yeast into acetaldehyde, with the release of CO2 (think sparkling wines), and then the anaerobic breakdown of the acetaldehyde into ethanol (alcohol). Acetic acid is also produced and can cause the wine to sour (turn to vinegar) if it develops in excess.
Although it can be simply enjoyed, the process of making wine from grapes is a demanding process. It is a delicate dance of storage, temperature, yeast and oxygen (or lack thereof) with time. The flavor and the alcoholic strength depends greatly on the control the winemaker exercises over these variables. The primary reaction is the conversion of the natural sugars in the grapes by yeast into acetaldehyde, with the release of CO2 (think sparkling wines), and then the anaerobic breakdown of the acetaldehyde into ethanol (alcohol). Acetic acid is also produced and can cause the wine to sour (turn to vinegar) if it develops in excess.
Fir0002/Flagstaffotos - GFDL
Other volatile chemicals are also formed in the fermentation process. These chemicals include aldehydes, esters, ketones, terpenes and phenols and give each wine its own subtle and distinctive flavors and aromas. A well made wine is a complex chemical blend of these many ingredients which produces one of the most sophisticated tastes in the whole world. A well trained Sommelier can identify the type of wine, the grapes used, the region where they were grown and possibly even the vintage year just from "experiencing" the wine with all of his senses.
Jesus chose as His first miracle, at the request of His mother, to change water into wine. The master of the feast, the Sommelier if you will, tasted the wine and reported to the bridegroom, "you have kept the best until now! John 2:10 (NIV)."
There is more to wine than just the alcohol. Wine can be subtle but bold, a complex mixture of many distinctive flavors. When enjoyed with a well matched meal, it lightens and lifts the spirit. But when consumed just for its alcoholic content, it will eventually bring the body to destruction. Enjoy it responsibly.
Ephesians 5:18 - "Do not get drunk on wine, which leads to debauchery. Instead, be filled with the Spirit. (NIV)"
Fir0002/Flagstaffotos - GFDL
Other volatile chemicals are also formed in the fermentation process. These chemicals include aldehydes, esters, ketones, terpenes and phenols and give each wine its own subtle and distinctive flavors and aromas. A well made wine is a complex chemical blend of these many ingredients which produces one of the most sophisticated tastes in the whole world. A well trained Sommelier can identify the type of wine, the grapes used, the region where they were grown and possibly even the vintage year just from "experiencing" the wine with all of his senses.
Jesus chose as His first miracle, at the request of His mother, to change water into wine. The master of the feast, the Sommelier if you will, tasted the wine and reported to the bridegroom, "you have kept the best until now! John 2:10 (NIV)."
There is more to wine than just the alcohol. Wine can be subtle but bold, a complex mixture of many distinctive flavors. When enjoyed with a well matched meal, it lightens and lifts the spirit. But when consumed just for its alcoholic content, it will eventually bring the body to destruction. Enjoy it responsibly.
Ephesians 5:18 - "Do not get drunk on wine, which leads to debauchery. Instead, be filled with the Spirit. (NIV)"
Wednesday, October 12, 2011
Is God Left-Handed?
I am sure most of you have seen Michelangelo's "The Creation of Adam" Fresco in the Sistine Chapel where God reaches out with His right hand to touch the finger of Adam. Well I have it on good authority that God is ambidextrous if not left-handed. Did you know that all of the proteins in your body are made from only left-handed amino acid isomers?
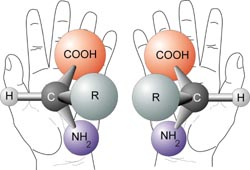
In chemistry, certain molecules with the same chemical formula can be formed in two or more configurations or isomers. In my last blog, I mentioned the "cis-" and "trans-" isomers of fatty acids. These are "Diastereomers", isomers that are not mirror images of each other. Enantiomers, sometimes called Optical Isomers, are mirror image isomers and cannot be superimposed on one another, much like your right and left hands. Your hands are the same but opposite much like the two optical isomers of an amino acid. This property of "handedness" is called Chirality.
Did you know that all of the proteins in your body are made from only left-handed amino acids?
Now in laboratory chemical reactions, the formation of a molecule that is chiral will result in a 50/50 mixture of the two isomers. But in the body, only one form, the L-amino acid is synthesized or used. This is because the function of a protein is determined by its shape and its shape is determined by the amino acid isomers in its sequence. If the protein consisted of mixed L- and D- amino acids, the protein could not fold properly and would not function as intended in our systems.
Now before you go and say that God is left-handed, lets look at the other major structures in our system, DNA and RNA. Proteins are derived from these two nucleic acids and the nucleotides that make up DNA and RNA are right-handed! Not only that but the DNA double helix spiral is also right-handed and the secondary structure of proteins is a right-handed coil called an alpha helix. So in the body, all of the amino acids are "left-handed' or L-amino acids and all of the nucleotides are "right-handed" D-nucleotides. God is indeed ambidextrous!
Mark 16:19 - "After the Lord Jesus had spoken to them, He was taken up into heaven and He sat at the right hand of God. (NIV)"
Sunday, October 9, 2011
Fats - Trans-lating the Lingo
There is a group of compounds that are a bane to all of us right now. They are fats. Our bodies take them in as a source of calories and for use in other metabolic processes and use them for long-term storage of energy as adipose tissue. But if we store too much, we can become overweight or obese and it is a health risk. In the USA, we are fat! 33% of all Americans are overweight and another 30% are downright fat! We see the words saturated, mono and polyunsaturated, and trans-fats thrown around on products everywhere telling us what to eat and what to avoid. What does it all mean?
Lets look at a typical fat. Fats consist of a number of different chemicals, most notably triglycerides and fatty acids and are part of a larger category called lipids. Fats can be solid (such as butter, shortening, lard or margarine), or liquid (such as any number of vegetable oils - olive, canola, peanut or corn). They can be saturated or unsaturated. Unsaturated fats can be mono (one) or poly (many) unsaturated and also be cis- or trans-isomers. Then we hear about omega-3 oils too!
Saturated fats are usually solid and have no carbon-carbon double bonds. Unsaturated fats are generally liquid and can be mono- or polyunsaturated - referring to the number of carbon-carbon double bonds that exist in the fatty acid chain. These double bonds can also be cis-isomer (the carbon chains are on the same side of the bond) or trans-isomer (the chains are on opposite sides). The cis-isomer is the naturally occurring unsaturated fat. Trans-isomers are created by chemical processing of foods and have a higher melting point than that of their cis-isomer cousins. They are also more difficult to metabolize.
Fats are essential in our diet. However, we need to consume fats in moderation and need to especially limit our intake of certain fats because they present a greater health risk. In general saturated fats are considered less healthy than unsaturated fats. Since saturated fats are solid at room temperature, they are less soluble in body fluids and can create globules in our system, which, with time, can clog our arteries resulting in strokes, heart attacks and embolisms. In general, the more unsaturated a fat is, the better it is for us. Thus poly-unsaturated fats were all of the rage years ago. Many products would boast of being higher in poly-unsaturated fats. These fats were more likely to be trans-fats. Now the emphasis is on limiting fats overall and trans-fats specifically. Trans-fats are by definition unsaturated and can be mono- or polyunsaturated. They are more likely to be solids and are not easily metabolized so they too increase the risk of heart attacks.
Another fat in the news is omega-3 Fish Oil. You may be taking a supplement from fish oil or other source with Omega-3 somewhere in the name. This refers to unsaturated fatty acids with the carbon double bond at the number 3 position (third carbon from the beginning of the chain). The fatty acids in these oils are essential fatty acids, primarily linolenic acid, which is used in numerous metabolic processes. Other essential fatty acids include linoleic acid, which is an omega-6 fatty acid. These can both be obtained in sufficient quantities in a regular diet from vegetables and many common cooking oils such as canola, flax or safflower. Taking extra can be beneficial if your diet is low in these essential fats but only a small amount of these are needed each day, the recommended intake being under 2 grams.
The ingestion of too many calories as fats, carbohydrates (sugars) or protein without a corresponding amount of exercise will result in weight gain. Proper diet and physical activity are necessary to maintain good health. Keeping our weight under control is just one factor in an overall healthy lifestyle.
Job 15:27 - "Though he has covered his face with his fatness, And made his waist heavy with fat, (NKJV)"
Lets look at a typical fat. Fats consist of a number of different chemicals, most notably triglycerides and fatty acids and are part of a larger category called lipids. Fats can be solid (such as butter, shortening, lard or margarine), or liquid (such as any number of vegetable oils - olive, canola, peanut or corn). They can be saturated or unsaturated. Unsaturated fats can be mono (one) or poly (many) unsaturated and also be cis- or trans-isomers. Then we hear about omega-3 oils too!
| Types of Fats (Fatty Acids) | |
|---|---|
Saturated (Myristic Acid) | |
Cis- & Trans- | Fat Types
|
Saturated fats are usually solid and have no carbon-carbon double bonds. Unsaturated fats are generally liquid and can be mono- or polyunsaturated - referring to the number of carbon-carbon double bonds that exist in the fatty acid chain. These double bonds can also be cis-isomer (the carbon chains are on the same side of the bond) or trans-isomer (the chains are on opposite sides). The cis-isomer is the naturally occurring unsaturated fat. Trans-isomers are created by chemical processing of foods and have a higher melting point than that of their cis-isomer cousins. They are also more difficult to metabolize.
Fats are essential in our diet. However, we need to consume fats in moderation and need to especially limit our intake of certain fats because they present a greater health risk. In general saturated fats are considered less healthy than unsaturated fats. Since saturated fats are solid at room temperature, they are less soluble in body fluids and can create globules in our system, which, with time, can clog our arteries resulting in strokes, heart attacks and embolisms. In general, the more unsaturated a fat is, the better it is for us. Thus poly-unsaturated fats were all of the rage years ago. Many products would boast of being higher in poly-unsaturated fats. These fats were more likely to be trans-fats. Now the emphasis is on limiting fats overall and trans-fats specifically. Trans-fats are by definition unsaturated and can be mono- or polyunsaturated. They are more likely to be solids and are not easily metabolized so they too increase the risk of heart attacks.
Another fat in the news is omega-3 Fish Oil. You may be taking a supplement from fish oil or other source with Omega-3 somewhere in the name. This refers to unsaturated fatty acids with the carbon double bond at the number 3 position (third carbon from the beginning of the chain). The fatty acids in these oils are essential fatty acids, primarily linolenic acid, which is used in numerous metabolic processes. Other essential fatty acids include linoleic acid, which is an omega-6 fatty acid. These can both be obtained in sufficient quantities in a regular diet from vegetables and many common cooking oils such as canola, flax or safflower. Taking extra can be beneficial if your diet is low in these essential fats but only a small amount of these are needed each day, the recommended intake being under 2 grams.
The ingestion of too many calories as fats, carbohydrates (sugars) or protein without a corresponding amount of exercise will result in weight gain. Proper diet and physical activity are necessary to maintain good health. Keeping our weight under control is just one factor in an overall healthy lifestyle.
Job 15:27 - "Though he has covered his face with his fatness, And made his waist heavy with fat, (NKJV)"
Friday, October 7, 2011
The Period I & II Elements - Some Interesting Scientific Facts
The world is full of many fascinating facts, in all kinds of arenas. Lets look at a few basic scientific facts relating to some of the elements in the first two periods, Hydrogen to Neon. These elements are the fundamental building blocks of our existence and it is the unique properties of these elements that are the basis for many of the different compounds we know. Here are just a few interesting facts about these elements and a few of the simpler compounds they make.
"Now faith is the substance of things hoped for, the evidence of things not seen. Hebrews 11:1 (NKJV)"
- Hydrogen is the simplest, least dense and most abundant element in the universe.
- Hydrogen consists of one proton and one electron.
- Hydrogen accounts for approximately 75% of all the physical mass in the universe, mostly found in stars.
- Hydrogen is believed to be one of only three elements produced in the Big Bang; the other two are helium and lithium.
- Hydrogen consists of one proton and one electron.
- Diamonds are the hardest substance known to man.
- Most gemstones contain several elements. The exception? The diamond. It is all carbon.
- A diamond will not dissolve in acid. The only thing that can destroy it is intense heat.
- Graphite, one of the softest of substances, is also all carbon and one of its most stable forms.
- Most gemstones contain several elements. The exception? The diamond. It is all carbon.
- Boron nitride (BN) is the second hardest substance known to man.
- Boron is element 5, one less than carbon (6).
- Nitrogen is element 7, one more than carbon.
- Boron nitride also exists in a soft, graphite-like form.
- Boron is element 5, one less than carbon (6).
- Oxygen, carbon, hydrogen and nitrogen make up over 95% of the human body.
- Oxygen (65.0%)
- Carbon (18.5%)
- Hydrogen (9.5%)
- Nitrogen (3.2%)
- Oxygen (65.0%)
- Hydrofluoric acid (HF) will dissolve glass.
- Hydrofluoric acid dissolves many oxides, of which glass (mainly quartz - SiO2) is just one.
- Other acids, such as hydrochloric acid, nitric acid and sulfuric acid, do not dissolve glass.
- Hydrofluoric acid must be stored in plastic containers.
- Hydrofluoric acid dissolves many oxides, of which glass (mainly quartz - SiO2) is just one.
"Now faith is the substance of things hoped for, the evidence of things not seen. Hebrews 11:1 (NKJV)"
Tuesday, October 4, 2011
Why I Believe Genesis 1:1-2
I believe that God created the heavens and the earth. Is this contrary to Science? I don't think so. The Oxford English Dictionary defines the scientific method as: "a method of procedure that consists of systematic observation, measurement, and experiment, and the formulation, testing, and modification of hypotheses." Science tries to prove something by direct observation and experimentation. When something cannot be seen, science will put forth a theory and then will test the theory against the available evidence until it can be "proven" if it can not be directly observed.
The creation of the universe, the "Big Bang" if you will, cannot be directly observed. Science is trying to do that by looking into deep space, back billions of years, seeing light from the edge of the universe, to show that the universe started from this massive explosion of a "singularity" into the universe we know today. Using physical laws and theories, science has shown that this is the best current explanation or "belief" for the origin of the universe, but it does not fit all of the laws and theories exactly so there is still room for speculation. Many also believe that Science does not allow room for God since God cannot be directly observed using a scientific method.
I do believe that the "Big Bang" is a plausible origin of the heavens as we know it. But what caused the Big Bang? Was it due to the sheer laws of physics coming together explosively or did it happen from the mind of an all knowing, all-powerful Creator? I don't believe this can be answered scientifically and that either "theory" takes faith. To me the theory of a Creator God makes more sense than an explosion due to the pressure of physical laws, especially since those laws most likely did not exist prior to the explosion. It seems to me that before the Big Bang, there were no physical laws because there was no physical reality. Physical reality came into existence the instant that the explosion happened and not a nanosecond before. God, on the other hand, would be outside of the space-time continuum that is our present reality and so could bring to bear such an event.
Looking at Genesis 1:1-2 it reads: "In the beginning God created the heavens and the earth. The earth was without form, and void; and darkness was on the face of the deep. And the Spirit of God was hovering over the face of the waters. (NKJV)"
Now if we look at the Hebrew words from the original text we find that some have multiple meanings. The words in Genesis 1:1 could mean:
And similarly for Genesis 1:2, the words could mean:
And thus Genesis 1:1-2 could be paraphrased:
Genesis 1:1 In the beginning God created the universe and all physical matter. 1:2 And all matter came into existence without elemental structure, and emptiness; and darkness filled the entirety of space. And the breathe of God blew throughout the presence the transitory elements.
Now I am not a Hebrew scholar so I may be stretching the meanings somewhat but I think you get the idea. Genesis 1:1-2 seems to be in line with a creation similar to what scientists are proposing with the Big Bang theory. Genesis 1:1 could be the singularity of the "Big Bang" and Genesis 1:2 could describe the explosion at the moment of creation. The Creation as outlined in Genesis 1:1-2 supports the scientific evidence of the Big Bang and vice versa.
I believe there is something that Science cannot "prove" and that is the existence of God. So you need to have faith to believe in Him or faith to believe He does not exist. There are many signs that He does exist, such as those espoused by Creation Scientists of which I consider myself one - Intelligent Design and Irreducible Complexity (see Reasons to Believe) being two, but since God is not directly observable many scientists will write Him out totally. I think if you look at the evidence for God, you must conclude He is real, that He directly touches our existence and that He has created us for a reason with a purpose.
Isaiah 40:21-22 "Have you not known? Have you not heard? Has it not been told you from the beginning? Have you not understood from the foundations of the earth? It is He who sits above the circle of the earth, And its inhabitants are like grasshoppers, He who stretches out the heavens like a curtain, And spreads them out like a tent to dwell in. (NKJV)"
The creation of the universe, the "Big Bang" if you will, cannot be directly observed. Science is trying to do that by looking into deep space, back billions of years, seeing light from the edge of the universe, to show that the universe started from this massive explosion of a "singularity" into the universe we know today. Using physical laws and theories, science has shown that this is the best current explanation or "belief" for the origin of the universe, but it does not fit all of the laws and theories exactly so there is still room for speculation. Many also believe that Science does not allow room for God since God cannot be directly observed using a scientific method.
I do believe that the "Big Bang" is a plausible origin of the heavens as we know it. But what caused the Big Bang? Was it due to the sheer laws of physics coming together explosively or did it happen from the mind of an all knowing, all-powerful Creator? I don't believe this can be answered scientifically and that either "theory" takes faith. To me the theory of a Creator God makes more sense than an explosion due to the pressure of physical laws, especially since those laws most likely did not exist prior to the explosion. It seems to me that before the Big Bang, there were no physical laws because there was no physical reality. Physical reality came into existence the instant that the explosion happened and not a nanosecond before. God, on the other hand, would be outside of the space-time continuum that is our present reality and so could bring to bear such an event.
Looking at Genesis 1:1-2 it reads: "In the beginning God created the heavens and the earth. The earth was without form, and void; and darkness was on the face of the deep. And the Spirit of God was hovering over the face of the waters. (NKJV)"
Now if we look at the Hebrew words from the original text we find that some have multiple meanings. The words in Genesis 1:1 could mean:
- Created = formed, shaped or fashioned
- Heavens = visible universe
- Earth = physical matter (as opposed to space)
And similarly for Genesis 1:2, the words could mean:
- Earth = physical matter
- Was = came into existence
- Without form = a place of chaos, lack of elemental form as we know it today
- Void = emptiness
- On, over = throughout, filled
- Face = presence, sight, in front of
- Deep = deep place = space
- Spirit = breath, wind, blast
- God = works of God
- Hovering = moving, shaking
- Waters = violence, transitory things
And thus Genesis 1:1-2 could be paraphrased:
Genesis 1:1 In the beginning God created the universe and all physical matter. 1:2 And all matter came into existence without elemental structure, and emptiness; and darkness filled the entirety of space. And the breathe of God blew throughout the presence the transitory elements.
Now I am not a Hebrew scholar so I may be stretching the meanings somewhat but I think you get the idea. Genesis 1:1-2 seems to be in line with a creation similar to what scientists are proposing with the Big Bang theory. Genesis 1:1 could be the singularity of the "Big Bang" and Genesis 1:2 could describe the explosion at the moment of creation. The Creation as outlined in Genesis 1:1-2 supports the scientific evidence of the Big Bang and vice versa.
I believe there is something that Science cannot "prove" and that is the existence of God. So you need to have faith to believe in Him or faith to believe He does not exist. There are many signs that He does exist, such as those espoused by Creation Scientists of which I consider myself one - Intelligent Design and Irreducible Complexity (see Reasons to Believe) being two, but since God is not directly observable many scientists will write Him out totally. I think if you look at the evidence for God, you must conclude He is real, that He directly touches our existence and that He has created us for a reason with a purpose.
Isaiah 40:21-22 "Have you not known? Have you not heard? Has it not been told you from the beginning? Have you not understood from the foundations of the earth? It is He who sits above the circle of the earth, And its inhabitants are like grasshoppers, He who stretches out the heavens like a curtain, And spreads them out like a tent to dwell in. (NKJV)"