A single atom represents one of the 92 natural Elements on the Periodic Table. Each atom consists of a varying combination of three main components, Protons, Neutrons and Electrons. Although science has identified many other particles inside the atom, for these discussions these three will be all we need to know.
Here is a picture of a Helium atom.
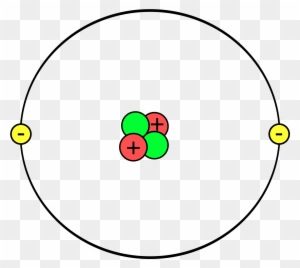
Public Domain
An atom can be likened to a golf ball. One type of golf ball consists of a small core, usually liquid filled, surrounded by a large wrapping of rubber bands and covered with a thin shell. The liquid filled core of the golf ball represents the nucleus of the atom. This is made up of protons and neutrons. The rubber band and shell represents the electron cloud that circles and encloses the atom. The electrons move around the nucleus so fast so as not to be identified as to their exact location. They are represented by a cloud. Just as the rubber band winds continuously around the core of the golf ball, so too, the electrons wrap around the nucleus. Depending on the number of electrons and their energy state, there are different defined orbits in which they reside.
A bucket full of identical golf balls would be similar to a bucket containing a single element. If you could see the individual atoms of the element, each one would be independent of all of the others, much like the golf balls.
An individual atom is characterized by the number of protons it contains. All atoms in an element, defined as a substance consisting of only one type of atom, have the same number of protons. The number of neutrons may vary in these atoms but the element will still be the same. Normally the number of neutrons is the same or almost the same as the number of protons. When the number of neutrons vary between similar atoms in a specific element, the different atoms are "isotopes" of that element. An isotope is still the same element but may have some different properties. An example of this is the element Carbon. Carbon has 6 protons in each of its atoms. It can have different numbers of neutrons. In carbon, the most common number of neutrons is 6 but it can also be 7 or 8. Carbon-14 (a isotope of carbon with 6 protons and 8 neutrons) can be used to date fossils as it decays back to carbon-12 at a known rate, thus allowing scientists to determine approximately the age of the fossil.s are organized into a chart or table called the "Periodic Table". Here they are listed by their "atomic number" or number of protons but are placed in rows and columns based on their "period" which is the number of shells occupied by their electrons. The first row, or shell, has 2 electrons, the second 8 electrons and on up in successive shells to the 118 elements currently listed. The number and shell of the outermost electrons plays a big role in the physical properties of the element.
No comments:
Post a Comment